Multiple Choice
The nuclear charge for an atom of 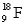 is
is
A) zero
B) + 9
C) + 18
D) + 28
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q48: After the 4s subshell of an atom
Q49: Isotopes of a given element<br>A) have the
Q50: Characterize EACH of the following statements as
Q51: Period number and group number in the
Q52: Choose the appropriate electron configuration for an
Q54: Which of the following elements is a
Q55: Choose the correct atom that has a
Q56: Characterize EACH of the following statements as
Q57: Choose the appropriate pair of atoms that
Q58: Which of the following statements is correct