Multiple Choice
In the acid-base reaction: HCN(aq) + F-(aq) 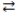 CN-(aq) + HF(aq) , where Ka (HCN) = 4.9 × 10-10 and Ka (HF) = 7.2 × 10-4, the
CN-(aq) + HF(aq) , where Ka (HCN) = 4.9 × 10-10 and Ka (HF) = 7.2 × 10-4, the
A) products are favored.
B) reactants are favored.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q2: All compounds can be classified as either
Q10: A solution containing a low concentration HCl
Q15: Although a Brønsted-Lowry acid must contain a
Q43: Which diagram properly depicts the dissolved species
Q50: A Brønsted-Lowry acid is _.<br>A)a compound that
Q57: A salt derived from a strong base
Q60: Normal gastric juice has a pH of
Q75: Most acids are weak acids.
Q87: The pH of normal blood is 7.4.
Q99: Which acid has the strongest conjugate base?<br>A)Hydrogen