Multiple Choice
What is the balanced equation for the acid-base reaction of nitrous acid with lithium hydroxide?
A) LiOH(aq) + HNO3(aq) 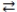 H2O(l) + LiNO3(aq)
H2O(l) + LiNO3(aq)
B) Li(OH) 2(aq) + 2 HNO3(aq) 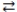 2 H2O(l) + Li(NO3) 2(aq)
2 H2O(l) + Li(NO3) 2(aq)
C) LiOH(aq) + HNO2(aq) 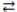 H2O(l) + LiNO2(aq)
H2O(l) + LiNO2(aq)
D) 2 LiOH(aq) + H2NO2(aq) 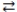 2 H2O(l) + Li2NO2(aq)
2 H2O(l) + Li2NO2(aq)
Correct Answer:

Verified
Correct Answer:
Verified
Q1: A solution in which [<sup>-</sup>OH] = 6.3
Q6: In the acid-base reaction: F<sup>-</sup>(aq)+ HNO<sub>3</sub>(aq) <img
Q8: What is the expression for K<sub>w</sub>, the
Q9: What is the pH of a peach
Q14: In a reversible acid-base reaction,equilibrium favors the
Q34: OH<sup>-</sup> and NH<sub>4</sub><sup>+</sup> are both examples of
Q36: The pH of a 1.0 × 10<sup>-5</sup>
Q39: Which species is the conjugate base of
Q51: Each day,the stomach produces 2.0 L of
Q76: Which solution has the highest pH?<br>A)4.3 ×