Multiple Choice
What is the molarity of an HNO3 solution if 24.1 mL of a 0.250 M Ba(OH) 2 solution are needed to titrate a 15.0 mL sample of the acid according to the equation below? Ba(OH) 2(aq) + 2 HNO3(aq) 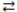 2 H2O(l) + Ba(NO3) 2(aq)
2 H2O(l) + Ba(NO3) 2(aq)
A) 0.402 M HNO3
B) 0.156 M HNO3
C) 0.311 M HNO3
D) 0.201 M HNO3
E) 0.803 M HNO3
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q9: Two species that differ by the presence
Q26: A sample of urine has a pH
Q26: Which species can act as a Brønsted-Lowry
Q30: What is the net ionic equation
Q57: A salt derived from a strong base
Q82: A strong acid readily donates a proton,forming
Q88: Ions that appear on both sides of
Q93: An aqueous solution of KCl has a
Q103: In an acid-base reaction,a proton is transferred
Q106: All salts where the anion is derived