Multiple Choice
When phosphoric acid (H3PO4) dissolves in water, the equilibrium shown below is established. If Ka = 7.5 × 10-3 for H3PO4, which statement concerning an aqueous solution of phosphoric acid is correct?
H3PO4 + H2O 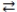 H3O+ + H2PO4-
H3O+ + H2PO4-
A) An aqueous solution of phosphoric acid contains mainly H3PO4 molecules.
B) An aqueous solution of phosphoric acid contains predominantly H3O+ and H2PO4- ions.
C) An aqueous solution of phosphoric acid contains equal amounts of H3O+ and H3PO4.
D) An aqueous solution of phosphoric acid contains a greater concentration of dissolved ions than it does neutral phosphoric acid molecules.
Correct Answer:

Verified
Correct Answer:
Verified
Q24: Which acid is the strongest?<br>A)Hydrogen sulfate ion
Q30: Which species is the conjugate acid of
Q35: What is the pH of a buffer
Q38: The addition of a base to water
Q59: A sample of water from the Chesapeake
Q67: The reaction of a Brønsted-Lowry acid (HA)with
Q69: The stronger the acid,the (larger/smaller)_ the value
Q70: An aqueous solution of NaHCO<sub>3</sub> is basic.
Q77: A Brønsted-Lowry base must contain a lone
Q108: A compound can be an acid or