True/False
In the reaction: SO32-(aq)+ H2O(l) 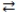 HSO3-(aq)+ -OH(aq), H2O acts as a Brønsted-Lowry acid.
HSO3-(aq)+ -OH(aq), H2O acts as a Brønsted-Lowry acid.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q11: Ammonia,NH<sub>3</sub>,is an example of a _.<br>A)strong acid<br>B)strong
Q21: Water is amphoteric; it can act as
Q28: An aqueous solution containing an equal number
Q31: An aqueous solution whose pH is greater
Q33: For a solution labeled 0.15 M CH<sub>3</sub>COOH,the
Q45: Which term correctly describes the medical condition
Q48: Which buffer solution has the lowest pH
Q52: The products are favored in the acid-base
Q72: The addition of an acid to water
Q80: Which salt forms a basic solution when