True/False
The reactants are favored in the acid-base reaction: HF(aq)+ -OH(aq) 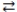 F-(aq)+ H2O(l).
F-(aq)+ H2O(l).
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q1: A solution in which [<sup>-</sup>OH] = 6.3
Q5: What is the pH of a cleaning
Q8: What is the expression for K<sub>w</sub>, the
Q9: What is the pH of a peach
Q13: How many milliliters of 0.653 M
Q15: The pH of a 1.0 × 10<sup>-6
Q34: OH<sup>-</sup> and NH<sub>4</sub><sup>+</sup> are both examples of
Q36: The pH of a 1.0 × 10<sup>-5</sup>
Q45: A compound that contains both a hydrogen
Q48: The value of K<sub>w</sub> = 1.0 ×