Multiple Choice
The Lewis structure shown below is not a valid Lewis structure. What statement best describes the error in the structure? 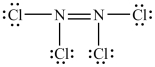
A) The nitrogen atoms violate the octet rule.
B) The chlorine atoms violate the octet rule.
C) The structure contains an incorrect number of valence electrons.
D) Chlorine atoms and nitrogen atoms do not typically form bonds with each other.
Correct Answer:

Verified
Correct Answer:
Verified
Q1: The Lewis structure for N<sub>2</sub>Cl<sub>4</sub> is _.
Q3: Carbon tetrachloride has _ valence electrons.
Q5: Which of the following is classified as
Q6: The symbol <span class="ql-formula"
Q9: Which statement concerning chemical bonds is false?<br>A)A
Q10: When writing Lewis structures, the symbol below
Q36: In the valence shell electron pair repulsion
Q38: Which molecule's Lewis structure contains an atom
Q42: The Lewis structure for PH<sub>3</sub> contains an
Q77: Unequal sharing of electrons in a covalent