Multiple Choice
Considering the electronegativity values indicated for each element, which covalent bond has the least degree of polarity? 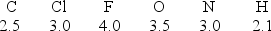
A) C-N
B) N-O
C) F-F
D) H-Cl
Correct Answer:

Verified
Correct Answer:
Verified
Q19: A molecule that contains only one polar
Q36: What is the molecular shape around the
Q37: Which molecule or ion has only two
Q38: Which bond is the most polar?<br>A)C-N<br>B)C-O<br>C)C-C<br>D)C-Cl<br>E)C-F
Q40: C-H bonds are considered to be nonpolar,because
Q46: Which of the following molecule(s)is(are)polar?<br>A)CO<sub>2</sub><br>B)CH<sub>4</sub><br>C)CBr<sub>4</sub><br>D)CHBr<sub>3</sub><br>E)More than one
Q58: The Lewis structure for BH<sub>3</sub> contains an
Q61: Resonance structures for a substance differ only
Q79: There can be a no more than
Q84: Atoms with seven valence electrons typically form