Multiple Choice
Which bonding pattern is NOT typical of carbon atoms in organic compounds?
A) 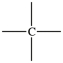
B) 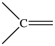
C) 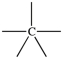
D) 
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q15: In order to complete the structure shown
Q16: The molecule with the condensed formula (CH<sub>3</sub>)<sub>2</sub>CHCH<sub>2</sub>CHO
Q17: The polarity in CH<sub>2</sub>=CHF can be represented
Q18: The two structures shown below represent the
Q19: Which represents a condensed structure for a
Q21: Vitamin D4 (structure shown)is a (fat/water)_-soluble vitamin.
Q23: How many lone pairs of electrons are
Q24: In terms of type of compound, the
Q25: Which structure properly indicates the order of
Q32: _ are organic compounds needed in small