Multiple Choice
Three of the four structures below represent unstable organic compounds that are not likely to exist because they violate the octet rule. Which one of the four structures represents a stable organic compound that is likely to exist?
A) 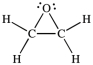
B) 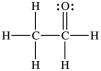
C) 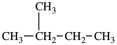
D) 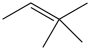
Correct Answer:

Verified
Correct Answer:
Verified
Q6: The compound whose skeletal structure is shown
Q7: How many lone pairs of electrons are
Q8: The molecule below contains alkyne and carbonyl
Q9: Which compound is an amide?<br>A) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5866/.jpg"
Q13: Cholesterol is soluble in the nonpolar solvent
Q14: Three of the four structures below represent
Q15: In order to complete the structure shown
Q16: The molecule with the condensed formula (CH<sub>3</sub>)<sub>2</sub>CHCH<sub>2</sub>CHO
Q75: CH<sub>2</sub>F<sub>2</sub> is a nonpolar molecule.
Q88: Organic compounds exist as discrete molecules with