True/False
The compound represented by the skeletal structure below contains eleven (11)H atoms and two lone pairs of electrons that are not shown. 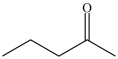
Correct Answer:

Verified
Correct Answer:
Verified
Q11: Which formula represents an organic compound?<br>A)NaCl<br>B)BaSO<sub>4</sub><br>C)PH<sub>3</sub><br>D)C<sub>4</sub>H<sub>10</sub>
Q40: Vitamin D4 (structure shown)is a (fat/water)_-soluble vitamin.
Q41: The compound below contains no polar bonds.
Q42: What is the shape about the O
Q43: Which represents a skeletal structure for a
Q47: The compound below is classified as what
Q48: What is the shape around each carbon
Q49: Which compound is an ether?<br>A) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5866/.jpg"
Q112: Which compound is most flammable?<br>A)HOCH<sub>2</sub>CH<sub>2</sub>OH<br>B)NaCl<br>C)CO<sub>2</sub><br>D)HCl
Q116: VSEPR theory is based on the concept