True/False
In the molecule below, the shape around the carbon atom is tetrahedral and the shape around the nitrogen atom is trigonal planar. 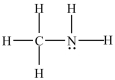
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q9: A covalent bond in which the electrons
Q57: Which representation has the bond polarities properly
Q58: Which compound is an aldehyde?<br>A) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5866/.jpg"
Q59: How many H atoms are bonded to
Q60: The molecule below is an example of
Q63: Which molecule is a polar molecule?<br>A) <img
Q64: What is the condensed formula for the
Q65: What is the most common multiple bond
Q66: Which compound is an amide?<br>A) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5866/.jpg"
Q67: In order to complete the structure below,