Multiple Choice
Use the following to answer questions
Scenario I
Scenario I is based on fabricated data inspired by the following study:
Pande, A. C., Crockatt, J. C., Feltner, D. E., et al. (2003) . Pregabalin in generalized anxiety disorder: a placebo-controlled trial. The American Journal of Psychiatry, 160(3) , 533-540.
Effect of Pregabalin on Anxiety
Pande and colleagues examined whether pregabalin (brand name Lyrica) was as effective as lorazepam (brand name Ativan) in treating anxiety. To that end, they administered either a low dose of pregabalin, a high dose of pregabalin, lorazepam, or placebo to participants for four weeks, then measured the participants' anxiety using the Hamilton Anxiety Rating Scale. The researchers found that not only did the high dose of pregabalin significantly reduce anxiety, but that it also reduced anxiety as well as lorazepam. The results of the study (Figure 1) indicate that pregabalin may be an effective alternative to lorazepam to treat anxiety in adults.
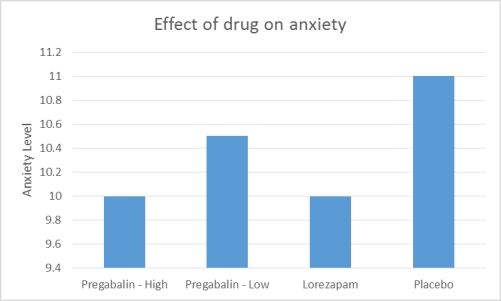 Figure 1. Hypothetical results of Pande et al (2003) showing the impact of each treatment on anxiety level. Anxiety was measured using the Hamilton Anxiety Rating Scale.
Figure 1. Hypothetical results of Pande et al (2003) showing the impact of each treatment on anxiety level. Anxiety was measured using the Hamilton Anxiety Rating Scale.
-(Scenario I) What ethical principle would be in question if the criteria for participating in the study included having health insurance?
A) beneficence
B) justice
C) respect for persons
D) It would violate all principles.
Correct Answer:

Verified
Correct Answer:
Verified
Q15: With regard to participants' emotional responses to
Q54: Minimal risk is to expedited review as:<br>A)
Q57: Loss of confidentiality is:<br>A) when the responses
Q58: Why might researchers fail to disclose the
Q60: Marsha would like to examine self-control in
Q61: Anonymity is:<br>A) when the responses and behaviors
Q64: Anthony is evaluating an IRB protocol that
Q114: Autonomy is crucial to which principle?<br>A) beneficence<br>B)
Q148: How might a research participant behave unethically?<br>A)
Q154: Representing others' work or ideas as your