Multiple Choice
You have a solution of 0.10 M Cl- and 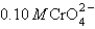 . You add 0.10 M silver nitrate dropwise into the solution. Ksp for Ag2CrO4 is 9.0 10-12 and for AgCl is1.6 10-10. Which of the following will precipitate first?
. You add 0.10 M silver nitrate dropwise into the solution. Ksp for Ag2CrO4 is 9.0 10-12 and for AgCl is1.6 10-10. Which of the following will precipitate first?
A) silver nitrate
B) silver chromate
C) cannot be determined from the information given
D) silver chloride
E) none of these
Correct Answer:

Verified
Correct Answer:
Verified
Q28: Equal volumes of 0.1 M HCl and
Q38: The two salts AgX and AgY have
Q110: Explain how the solubility of an ionic
Q142: A 200-mL solution contains 0.018 mol each
Q143: After adding 25.0 mL of 0.100 M
Q145: Consider the titration of 200.0 mL of
Q146: Which of the following solutions will be
Q149: 11.8 mL of 0.30 M HCl is
Q150: What is the solubility of Mg(OH)<sub>2</sub> (K<sub>sp</sub>
Q151: Silver acetate (AgC<sub>2</sub>H<sub>3</sub>O<sub>2</sub>) is a sparingly soluble