Multiple Choice
A 50.0-mL sample of 2.0 10-4 M CuNO3 is added to 50.0 mL of 4.0 M NaCN. Cu+ reacts with CN- to form the complex ion Cu(CN) 32-: 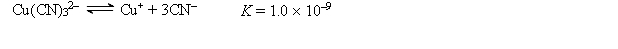 What is the concentration of CN- at equilibrium?
What is the concentration of CN- at equilibrium?
A) 6.0 10-4 M
B) 1.0 M
C) 4.0 M
D) 2.0 M
E) none of these
Correct Answer:

Verified
Correct Answer:
Verified
Q16: Derive the Henderson-Hasselbalch equation from the K<sub>a</sub>
Q34: A solution contains 0.34 M HA (K<sub>a</sub>
Q35: Methyl orange is an indicator with a
Q36: In the titration of 100.0 mL of
Q37: Given the following K<sub>sp</sub> values <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6420/.jpg"
Q42: A 200.0-mL sample of the weak acid
Q44: The K<sub>sp</sub> value for PbSO<sub>4</sub><sub>(s) </sub>is 1.3
Q61: What is the molarity of a sodium
Q68: Differentiate between the equivalence point and the
Q81: Explain how to decide on a specific