Multiple Choice
A 50.0-mL sample of 2.0 10-4 M CuNO3 is added to 50.0 mL of 4.0 M NaCN. Cu+ reacts with CN- to form the complex ion Cu(CN) 32-: 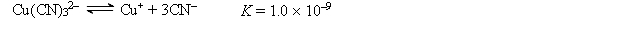 Calculate the solubility of CuBr(s) (Ksp = 1.0 10-5) in 1.0 L of 1.0 M NaCN.
Calculate the solubility of CuBr(s) (Ksp = 1.0 10-5) in 1.0 L of 1.0 M NaCN.
A) 0.33 mol/L
B) 1.0 10-6 mol/L
C) 1.0 103 mol/L
D) 1.0 mol/L
E) none of these
Correct Answer:

Verified
Correct Answer:
Verified
Q14: The solubility of Cd(OH)<sub>2</sub> in water is
Q15: A 200.0-mL sample of the weak acid
Q16: A solution containing 10. mmol of CO<sub>3</sub><sup>2</sup><sup>-</sup>
Q17: Calculate the pH when 200.0 mL of
Q18: Consider a solution of 2.0 M HCN
Q21: Which of the following compounds has the
Q22: A 0.012-mol sample of Na<sub>2</sub>SO<sub>4</sub> is added
Q23: One milliliter (1.00 mL) of acid taken
Q24: Titrating 30.00 mL of a saturated calcium
Q25: What quantity of NaOH(s) must be added