Essay
The cation M2+ reacts with NH3 to form a series of complex ions as follows: 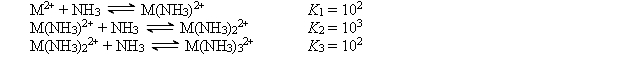 Consider an experiment in which 1.0 *10-3 mol of M(NO3)2 is added to 1.0 L of 15.0 M NH3. Calculate the equilibrium concentrations of M2+, M(NH3)22+, and M(NH3)32+
Consider an experiment in which 1.0 *10-3 mol of M(NO3)2 is added to 1.0 L of 15.0 M NH3. Calculate the equilibrium concentrations of M2+, M(NH3)22+, and M(NH3)32+
Correct Answer:

Verified
[M2+] = 3.0 *10-View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q106: Differentiate between a formation constant and a
Q109: A 50.00-mL sample of 0.100 M Ca(NO<sub>3</sub>)<sub>2</sub>
Q110: Which of the following titration curves schematically
Q111: The observed solubility of the salt MX
Q112: A solution is formed by mixing 50.0
Q115: What is the pH of a solution
Q116: Which of the following is the net
Q117: How many moles of Fe(OH)<sub>2</sub> [K<sub>sp</sub> =
Q118: How many moles of CaF<sub>2</sub> will dissolve
Q119: Calculate the solubility of Cu(OH)<sub>2</sub> in a