Multiple Choice
Which titration curve would result from the titration of phosphoric acid by a strong base?
A) 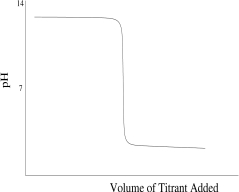
B) 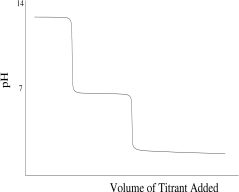
C) 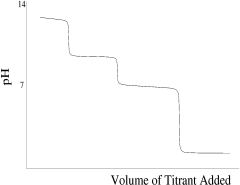
D) 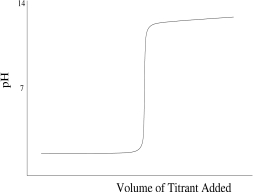
E) None of the above
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q101: The solubility of La(IO<sub>3</sub>)<sub>3</sub> in a 0.10
Q102: The concentration of Ag<sup>+</sup> in a saturated
Q103: Which of the following salts shows the
Q104: What is the molar solubility of AgCl
Q106: 44.4 mL of a 1.42 M NaOH
Q107: A titration of 100.0 mL of 1.00
Q108: You are given 5.00 mL of an
Q109: A 50.00-mL sample of 0.100 M Ca(NO<sub>3</sub>)<sub>2</sub>
Q110: Which of the following titration curves schematically
Q134: A student titrates an unknown weak acid,