Multiple Choice
What is the equilibrium constant for the following reaction? NH4+ + OH-  NH3 + H2O
NH3 + H2O
A) 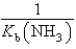
B) 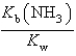
C) 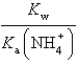
D) 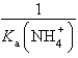
E) 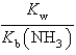
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q1: What concentration of HF (K<sub>a</sub> = 7.2
Q2: The equilibrium constant for the reaction A<sup>-</sup>
Q3: Calculate the pH of a 2.0 <font
Q4: As water is heated, its pH decreases.
Q8: Which of the following is the equilibrium
Q11: Calculate the pH of a solution that
Q43: The following acids are listed in order
Q67: If solid sodium cyanide (NaCN) is dissolved
Q78: Consider two separate solutions of equal concentration.
Q119: Calculate the pH of a 0.02 M