Multiple Choice
If Ka for HCN is 6.2 10-10, what is Kb for CN-? 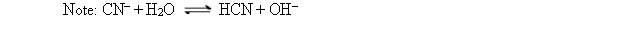
A) 6.2 10-24
B) 6.2 104
C) 1.6 10-5
D) 1.6 1023
E) none of these
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q61: Calculate the pH of the following aqueous
Q62: Calculate [H<sup>+</sup>] in a 1.0 M solution
Q63: For a neutral solution, it must be
Q65: A monoprotic weak acid, when dissolved in
Q67: Which of the following reactions is associated
Q68: Which reaction does not proceed far to
Q69: Of the bases NaOH, H<sub>2</sub>O, CN<sup>-</sup>, SO<sub>4</sub><sup>2</sup><sup>-</sup>,
Q70: Calculate the pOH of a 0.10 M
Q71: Calculate the pH of a 0.50 M
Q87: The sodium salt, NaA, of a weak