Multiple Choice
Samples of the gases H2(g) and SO2(g) have equal masses and are at the same temperature and pressure. Calculate the following:
-The ratio of the root-mean-square velocities 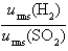 .
.
A) 180
B) 32
C) 0.18
D) 1.0
E) 5.6
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q14: Consider two samples of helium in separate
Q20: A 275.0-mL sample of O<sub>2</sub> is collected
Q42: The ratio V/n versus the Kelvin temperature
Q45: When a mixture is prepared from 15.0
Q47: A balloon contains an anesthetic mixture of
Q49: A balloon contains 10.0 g of neon
Q58: Magnesium metal reacts with hydrochloric acid to
Q70: Four identical 1.0-L flasks contain the gases
Q80: Body temperature is about 308 K. On
Q116: Which impurity in mined coal, when burned,