Multiple Choice
A complex ion is a square planar complex. It has a d8 electron configuration. What is the most reasonable d orbital scheme for this complex?
A) 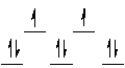
B) 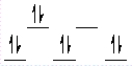
C) 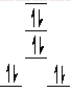
D) 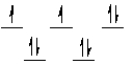
E) 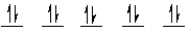
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q6: How many unpaired electrons are found in
Q8: Which of the following ligands are capable
Q9: How many unpaired electrons are found in
Q11: In which of the following complexes does
Q12: A metal ion in a high-spin octahedral
Q15: How many of the following compounds exhibit
Q16: How many unpaired electrons are found in
Q44: The color of a transition metal complex
Q50: How many unpaired electrons are there in
Q77: _ isomers and _ isomers are classes