Multiple Choice
A certain complex ion has a distorted octahedral structure in which the ligands along the plus and minus z axes are compressed (pushed in closer to the central metal ion) . The d orbital splitting diagram for this complex ion would be
A) 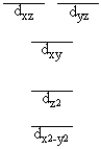
B) 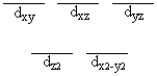
C) 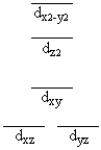
D) 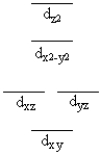
E) 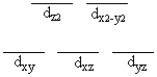
Correct Answer:

Verified
Correct Answer:
Verified
Q10: What is the maximum oxidation state of
Q22: A compound of which transition metal is
Q24: Here are some crystal field representations of
Q25: Which of the following complexes would be
Q28: The complex ion [TiBr<sub>4</sub>]<sup>2</sup><sup>-</sup> is tetrahedral. How
Q29: Explain how the molecular orbital model accounts
Q37: Which of the following complexes shows geometric
Q74: The complex ion Ni(NH<sub>3</sub>)<sub>6</sub><sup>2+</sup> (two unpaired electrons)
Q85: Explain the toxicities of carbon monoxide (CO)
Q96: Specify the number of unpaired electrons in