Multiple Choice
Here are some crystal field representations of d electrons in an octahedral field: Choose the representation that fits the transition metal atom in the following species.
-Fe(OH2) 63+ (assume weak field)
A) 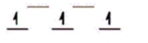
B) 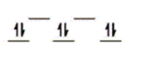
C) 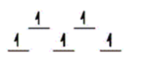
D) 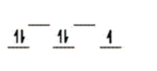
E) 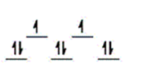
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q7: Transition metals show great similarities both within
Q9: Carboxyhemoglobin is formed when _ prevents the
Q24: A coordination compound of Cu<sup>2+</sup> can be
Q56: A d<sup>6</sup> ion (Fe<sup>2+</sup>) is complexed with
Q69: For which of the following metal ions
Q70: Which metal ion has a d<sup>6</sup> electron
Q71: The corrosion of which transition metal results
Q71: Which of the metal ions in the
Q75: The complex FeL<sub>6</sub><sup>2+</sup>, where L is a
Q89: Which transition metal can exist in all