Multiple Choice
Here are some crystal field representations of d electrons in an octahedral field: Choose the representation that fits the transition metal atom in the following species.
-K4Mn(CN) 6 (assume strong field)
A) 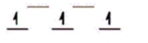
B) 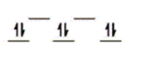
C) 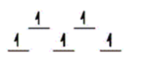
D) 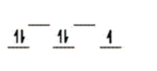
E) 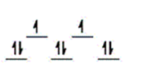
Correct Answer:

Verified
Correct Answer:
Verified
Q10: What is the maximum oxidation state of
Q12: A metal ion in a high-spin octahedral
Q15: How many of the following compounds exhibit
Q16: Fluoride ion ranks low in the spectrochemical
Q22: A compound of which transition metal is
Q29: Explain how the molecular orbital model accounts
Q37: Which of the following complexes shows geometric
Q44: The color of a transition metal complex
Q50: How many unpaired electrons are there in
Q74: The complex ion Ni(NH<sub>3</sub>)<sub>6</sub><sup>2+</sup> (two unpaired electrons)