Multiple Choice
Here are some crystal field representations of d electrons in an octahedral field: Choose the representation that fits the transition metal atom in the following species.
-K4Fe(CN) 6
A) 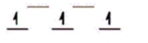
B) 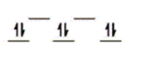
C) 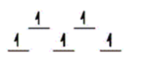
D) 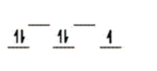
E) 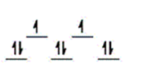
Correct Answer:

Verified
Correct Answer:
Verified
Q8: How many unpaired electrons are found in
Q41: Choose the most likely pattern for the
Q47: Which of the following are structural isomers?
Q48: The complex ion Fe(CN)<sub>6</sub><sup>4</sup><sup>-</sup> (no unpaired electrons)
Q50: How many unpaired electrons are there in
Q57: Use the crystal field model to explain
Q63: Which of the following coordination compounds will
Q83: The complex ions of Zn<sup>2+</sup> are all
Q87: The 3d electrons in Co(NH<sub>3</sub>)<sub>6</sub><sup>3+</sup> are all
Q90: The complex ion Co(NH<sub>3</sub>)<sub>6</sub><sup>2+</sup> (three unpaired electrons)