Multiple Choice
Acetone (mw = 58.08, P 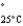 =232 mmHg) and butanone (mw = 72.11, P
=232 mmHg) and butanone (mw = 72.11, P 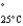 =100 mmHg) have the indicated molar masses and vapor pressures. A container holds 1.00 kg of butanone. How much acetone must be added to the butanone to elevate the total vapor pressure over the mixture to 125 mmHg at 25°C?
=100 mmHg) have the indicated molar masses and vapor pressures. A container holds 1.00 kg of butanone. How much acetone must be added to the butanone to elevate the total vapor pressure over the mixture to 125 mmHg at 25°C?
A) 5.313 kg
B) 290 g
C) 188 g
D) More information needed
E) None of the above
Correct Answer:

Verified
Correct Answer:
Verified
Q2: What is the molar mass of glucose
Q17: Predict the deviation from Raoult's law when
Q41: Predict the deviation from Raoult's law when
Q42: Diagram and label a vapor pressure diagram
Q53: The molal freezing-point depression constants for benzene
Q56: A 5.00-g sample of a compound is
Q70: The freezing-point depression of a 3.00 *10<sup>-</sup><sup>3</sup>
Q72: When 271.0 mL of 0.828 M HCl
Q79: A 5.96-g sample of a compound is
Q90: How many moles of methanol, CH<sub>3</sub>OH, dissolved