Multiple Choice
Aluminum metal crystallizes in a face-centered cubic structure. What is the relationship between the radius of an Al atom (r) and the length of an edge of the unit cell (E) ?
A) 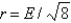
B) 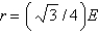
C) r = 2E
D) r = 4E
E) r = E/2
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q10: The triple point of a substance is<br>A)
Q17: Sodium oxide (Na<sub>2</sub>O) crystallizes in a structure
Q21: The molar volume of a certain form
Q22: Which of the following should have the
Q23: <font face="symbol"></font>H<sub>vap</sub> for water is 40.7 kJ/mol.
Q26: Which of the following substances would you
Q65: The molar volume of a certain form
Q80: Identify the major attractive force in HF.<br>A)
Q87: A liquid placed in a closed container
Q91: Chromium metal crystallizes as a body-centered cubic