Multiple Choice
A certain substance has the phase diagram shown below. At which of the following values of T and P is the substance a pure liquid? 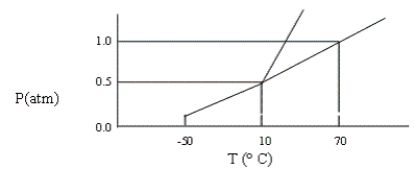
A) T = 70°C, P = 1.2 atm
B) T = 10°C, P = 1 atm
C) T = 8°C, P = 1 atm
D) T = 10°C, P = 0.5 atm
E) T = 80°C, P = 1 atm
Correct Answer:

Verified
Correct Answer:
Verified
Q2: Properties of liquids lie (closer to/further from)
Q9: Which substance involves no intermolecular forces except
Q10: The triple point of a substance is<br>A)
Q15: When 1.00 mol of a pure liquid
Q17: Sodium oxide (Na<sub>2</sub>O) crystallizes in a structure
Q32: Which substance can be described as cations
Q77: Which of the following processes must exist
Q83: In cubic closest-packed solids, what percentage of
Q87: A liquid placed in a closed container
Q89: The resistance of a liquid to an