Multiple Choice
Below is a phase diagram for compound X. You wish to purify a sample of X that was collected at P = 1.0 atm and T = 100 by subliming it. In order to sublime the sample, you should 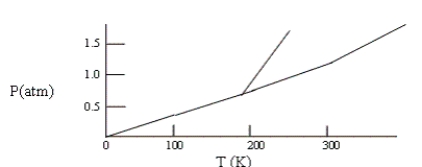
A) increase T to 300 K, keeping P = 1.0 atm.
B) abandon the attempt to sublime X.
C) lower P to 0.5 atm and then increase T to 200 K.
D) increase P to 1.5 atm and then increase T to 300 K.
E) increase T to 300 K and then lower P to 0.5 atm.
Correct Answer:

Verified
Correct Answer:
Verified
Q9: Which is generally larger, the heat of
Q33: A p-type semiconductor<br>A) is made by doping
Q44: Which of the following is the smallest
Q47: You are given a small bar of
Q88: How many grams of ice would be
Q91: Shown below is a phase diagram for
Q92: MnO has either a structure like NaCl
Q95: A certain compound with a molar mass
Q98: Below is a phase diagram for compound
Q107: What is the vapor pressure of water