Multiple Choice
Estimate the bond energy of the N2 molecule. 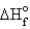 for NH3 = -46.0 kJ/mol N-H bond energy = 391 kJ/mol
for NH3 = -46.0 kJ/mol N-H bond energy = 391 kJ/mol
H-H bond energy = 432 kJ/mol
A) 1140 kJ/mol
B) 560 kJ/mol
C) 87 kJ/mol
D) 479 kJ/mol
E) 958 kJ/mol
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q20: Which of the following statements about the
Q23: In which of the following compounds does
Q51: For which compound is resonance required to
Q52: How many electrons are in the Lewis
Q55: Which of the following is nonpolar?<br>A) H<sub>2</sub>O<br>B)
Q60: Which of the following molecules has a
Q61: Which of the following has the Lewis
Q77: According to the VSEPR model, the electron
Q106: Which element listed below has the highest
Q125: Given the following Lewis structure: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6422/.jpg"