Multiple Choice
Given the following information: N2 bond energy = 941 kJ/mol
F2 bond energy = 154 kJ/mol 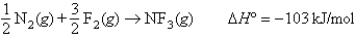 calculate the N-F bond energy.
calculate the N-F bond energy.
A) 113 kJ/mol
B) 268 kJ/mol
C) 66 kJ/mol
D) 317 kJ/mol
E) none of these
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q6: Which of the following molecules has a
Q9: Select the correct molecular structure for SO<sub>2</sub>.<br>A)
Q50: Select the correct molecular structure for IF<sub>6</sub><sup>+</sup>.<br>A)
Q60: Which of the following molecules has a
Q61: Which of the following has the Lewis
Q63: In which case is the bond
Q67: Which of the following groups contains no
Q110: For each of the following compounds:<br>A) Give
Q127: Which statement is correct?<br>A) H<sub>2</sub>O is linear.<br>B)
Q136: Use the following electronegativity values to answer