Multiple Choice
Given the following Lewis structure: 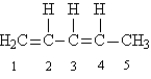
-How many electrons are shared between carbons 1 and 2?
A) 6
B) 2
C) 0
D) 8
E) 4
Correct Answer:

Verified
Correct Answer:
Verified
Q1: The molecule XCl<sub>5</sub><sup>-</sup> has a square pyramidal
Q2: Choose the molecule with the strongest bond.<br>A)
Q3: Choose the electron dot formula that most
Q4: In the Lewis structure for I<sub>3</sub><sup>-</sup>, there
Q6: Which of the following molecules has a
Q7: Which ion is planar?<br>A) CO<sub>3</sub><sup>2-</sup><br>B) SO<sub>3</sub><sup>2-</sup><br>C) PCl<sub>4</sub><sup>+</sup><br>D)
Q8: Draw the Lewis structures of the molecules
Q9: Select the correct molecular structure for SO<sub>2</sub>.<br>A)
Q10: Which of the following compounds contains only
Q11: Atoms having greatly differing electronegativities are expected