Multiple Choice
The energy expressions for the electrons in the He+ ion and the hydrogen atom are En (H) = -a/n2 and En (He+) = -4a/n2
Which of the following statements is(are) correct?
I. For the transitions 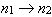 , the frequency is larger for H than for He+.
, the frequency is larger for H than for He+.
II. The first ionization energy of the H atom is smaller than the second.
III. The 1s orbital in He+ is larger (in the sense that the probability density is
shifted outward) than the 1s orbital in H.
A) III only
B) I and II only
C) I only
D) I, II, and III
E) II only
Correct Answer:

Verified
Correct Answer:
Verified
Q9: Given the electron configurations of the following
Q16: What is the electron configuration of indium?<br>A)
Q22: What is the electron configuration of Ti<sup>2+</sup>?<br>A)
Q52: A photographic film needs a minimum of
Q59: Write the electron configuration for the following:<br>-O<sup>2</sup><sup>-</sup>
Q60: Which of the following statements is(are) true?
Q74: An electron in a one-dimensional box requires
Q81: From the following list of observations, choose
Q85: The first ionization energy of Mg is
Q125: Which is the highest occupied energy orbital