Multiple Choice
The energy equation for a particle in a cubic box of dimensions Lx = Ly = Lz is Enx, ny, nz = 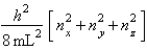 For a particle in a cubic box, how many degenerate energy levels have energy equal to 14 h2/8 mL2?
For a particle in a cubic box, how many degenerate energy levels have energy equal to 14 h2/8 mL2?
A) 1
B) 12
C) 6
D) 8
E) 3
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q78: How many unpaired electrons does chlorine have
Q83: Consider the following orderings.<br>I. Al < Si
Q92: For an electron in a 2.00-nm one-dimensional
Q98: The valence electron configuration of an element
Q110: How many electrons can be described by
Q111: Of the following elements, which needs 3
Q111: How many unpaired electrons are there in
Q112: Write the electron configuration for the following:<br>-Na
Q113: What is the wavelength of light that
Q120: How many unpaired electrons does manganese have