Multiple Choice
Which of the following combinations of quantum numbers is not allowed? (Combinations are listed as follows: n, l, m(l) , m(s) .)
A) 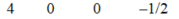
B) 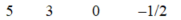
C) 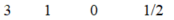
D)  .
.
E) 
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q7: For which element are the d orbitals
Q12: Consider the following portion of the energy-level
Q15: Given the electron configurations of the following
Q24: Which of the following frequencies corresponds to
Q30: Which of the following statements is false?<br>A)
Q31: Which of the following sets has elements
Q33: Which of the following atoms or ions
Q44: What is the electron configuration for Cr<sup>2+</sup>?<br>A)
Q99: In which orbital does an electron experience
Q103: Which of the following electron configurations is