Short Answer
The energy equation for a particle in a cubic box of dimensions Lx = Ly = Lz is
Enx, ny, nz = 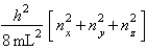 Assume that 8 electrons occupy the allowed energy levels and that 2 electrons can occupy each allowed energy level.
Assume that 8 electrons occupy the allowed energy levels and that 2 electrons can occupy each allowed energy level.
A)In the ground state, how many of the 8 electrons have energy equal to 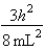 ?
?
B)In the ground state, how many of the 8 electrons have energy equal to 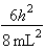 ?
?
C)In the ground state, how many of the 8 electrons have energy equal to 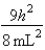 ?
?
D)Calculate the wavelength of light necessary to promote the highest-energy ground-state electron into the lowest-energy excited state. Assume a cubic box with dimensions 1.50 nm * 1.50 nm * 1.50 nm.
Correct Answer:

Verified
A) 2
B)6
C...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
B)6
C...
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q27: An element with the electron configuration [Xe]4f<sup>14</sup>5d<sup>7</sup>6s<sup>2</sup>
Q38: Which atom has three 2p electrons in
Q39: Light has a wavelength of 5.8 *10<sup>2</sup>
Q39: What is the total number of electrons
Q40: A photographic film needs a minimum of
Q60: From the following list of observations, choose
Q66: Given the electron configurations of the following
Q73: What is the electron configuration of Co<sup>3+</sup>?<br>A)
Q100: Which of the following statements is true
Q123: How many electrons can be described by