Multiple Choice
A complex ion is a square planar complex. It has a d8 electron configuration. What is the most reasonable d orbital scheme for this complex?
A) 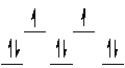
B) 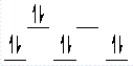
C) 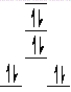
D) 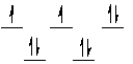
E) 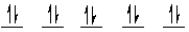
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q16: How many unpaired electrons are found in
Q17: Which of the following statements is true
Q18: The spectrochemical series is<br>I<sup>-</sup> < Br<sup>-</sup> <
Q19: Choose the most likely pattern for the
Q20: Specify the number of unpaired electrons in
Q22: A compound of which transition metal is
Q23: Which of the following statements is/are true
Q24: A coordination compound of Cu<sup>2+</sup> can be
Q25: In the Lewis acid-base model, acids are
Q26: In describing the bonding in coordination compounds