Multiple Choice
Which of the following crystal field diagrams is correct for Mn(CN) 63- , where CN- is a strong-field ligand?
A) 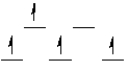
B) 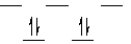
C) 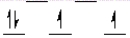
D) 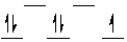
E) none of these
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q54: Which has the greater number of unpaired
Q55: For the process Co(NH<sub>3</sub>)<sub>5</sub>Cl<sup>2+</sup> + Cl<sup>-</sup> →
Q56: A d<sup>6</sup> ion (Fe<sup>2+</sup>) is complexed with
Q57: Use the crystal field model to explain
Q58: A metal ion in a high-spin octahedral
Q60: How many unpaired electrons are found in
Q61: The phenomenon called the _ contraction is
Q62: The complex ion [NiCl<sub>4</sub>]<sup>2-</sup> is tetrahedral. How
Q63: Which of the following coordination compounds will
Q64: What is the electron configuration of the