Multiple Choice
Here are some crystal field representations of d electrons in an octahedral field: Choose the representation that fits the transition metal atom in the following species.
-Fe(OH2) 63+ (assume weak field)
A) 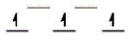
B) 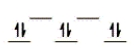
C) 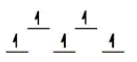
D) 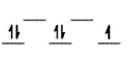
E) 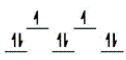
Correct Answer:

Verified
Correct Answer:
Verified
Q25: In the Lewis acid-base model, acids are
Q26: In describing the bonding in coordination compounds
Q27: How many of the following compounds exhibit
Q28: Which of the following is a d<sup>2</sup>
Q29: Explain how the molecular orbital model accounts
Q31: Which of the following is paramagnetic?<br>A) [Co(NH<sub>3</sub>)<sub>6</sub>]<sup>3+</sup>
Q32: Identify the true statement(s) of scandium.<br>1. The
Q33: Which of the following is a d<sup>7</sup>
Q34: Which of the metal ions in the
Q35: Which of the following statements is true