Multiple Choice
Here are some crystal field representations of d electrons in an octahedral field: Choose the representation that fits the transition metal atom in the following species.
-K4Mn(CN) 6 (assume strong field)
A) 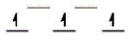
B) 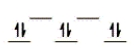
C) 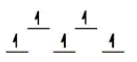
D) 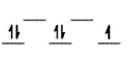
E) 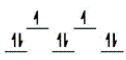
Correct Answer:

Verified
Correct Answer:
Verified
Q60: How many unpaired electrons are found in
Q61: The phenomenon called the _ contraction is
Q62: The complex ion [NiCl<sub>4</sub>]<sup>2-</sup> is tetrahedral. How
Q63: Which of the following coordination compounds will
Q64: What is the electron configuration of the
Q66: The principal electron transfer molecules in the
Q67: Specify the number of unpaired electrons in
Q68: Which of the following crystal field diagrams
Q69: How many unpaired electrons are found in
Q70: How many unpaired electrons are found in