Short Answer
Consider the pseudo-octahedral complex of Cr3+ shown below, where A and B represent Lewis bases and where A produces a stronger crystal field than B. Draw an appropriate crystal field diagram for this complex (include the electrons). 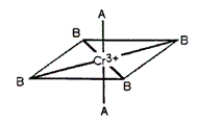
Correct Answer:

Verified
Correct Answer:
Verified
Q81: Analysis of the data from a titration
Q82: Addition of AgNO<sub>3</sub> to aqueous solutions of
Q83: The complex ions of Zn<sup>2+</sup> are all
Q84: For which of the following metal ions
Q85: Explain the toxicities of carbon monoxide (CO)
Q87: The 3d electrons in Co(NH<sub>3</sub>)<sub>6</sub><sup>3+</sup> are all
Q88: How many unpaired electrons are found in
Q89: Which transition metal can exist in all
Q90: The complex ion Co(NH<sub>3</sub>)<sub>6</sub><sup>2+</sup> (three unpaired electrons)
Q91: Give the number of geometric isomers for