Multiple Choice
Acetone (mw = 58.08, P 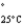 =232 mmHg) and butanone (mw = 72.11, P
=232 mmHg) and butanone (mw = 72.11, P 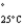
=100 mmHg) have the indicated molar masses and vapor pressures. A container holds 1.00 kg of butanone. How much acetone must be added to the butanone to elevate the total vapor pressure over the mixture to 125 mmHg at 25°C?
A) 5.313 kg
B) 290 g
C) 188 g
D) More information needed
E) None of the above
Correct Answer:

Verified
Correct Answer:
Verified
Q42: Diagram and label a vapor pressure diagram
Q43: Using the data below, calculate the vapor
Q44: You use 2.0 g of solid MX
Q45: Consider a solution containing liquids A and
Q46: A liquid-liquid solution is called an ideal
Q47: Liquid A has vapor pressure x. Liquid
Q49: A solution contains 1 mol of liquid
Q50: How many milliliters of 11.8 M HNO<sub>3</sub>
Q52: The freezing-point depression of a 3.00 ×
Q53: The molal freezing-point depression constants for benzene