Multiple Choice
A sample consisting of CO2(g) and CO2(s) at equilibrium at -78°C and 1 atm pressure is heated to -30°C, and the pressure is increased to 8 atm. Based on the phase diagram below, what will happen? 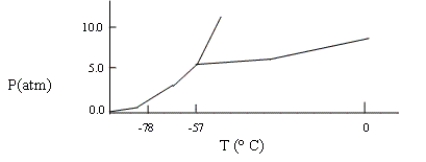
A) At equilibrium, only CO2(g) will be present.
B) The melting point of the CO2(s) will decrease.
C) At equilibrium, CO2(g) and CO2(l) will be present.
D) All the CO2 will be converted to CO2(l) .
E) none of these
Correct Answer:

Verified
Correct Answer:
Verified
Q31: Based on intermolecular forces, which of the
Q32: Which substance can be described as cations
Q33: A p-type semiconductor<br>A) is made by doping
Q34: Identify the major attractive force in Ne.<br>A)
Q37: A sample of Co crystallizes in the
Q38: When 1.00 mol of a pure liquid
Q39: A certain metal fluoride crystallizes in such
Q40: The unit cell in a certain lattice
Q40: In the unit cell of sphalerite, Zn<sup>2+</sup>
Q41: What is the vapor pressure of water