Multiple Choice
Shown below is a phase diagram for compound X. How will the melting point of X change with increased pressure? 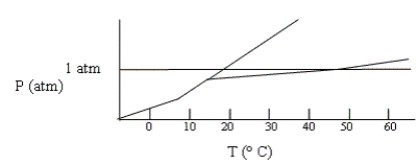
A) There is not enough information given.
B) It will increase.
C) It will increase and then decrease.
D) It will decrease.
E) It will remain the same.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q81: Shown below is a phase diagram for
Q82: Alkali halides commonly have either the sodium
Q83: In cubic closest-packed solids, what percentage of
Q84: Given the phase diagram shown below, which
Q85: A certain substance, X, has a triple-point
Q87: A liquid placed in a closed container
Q88: How many grams of ice would be
Q89: The resistance of a liquid to an
Q90: A metal crystallizes in a body-centered unit
Q91: Chromium metal crystallizes as a body-centered cubic