Multiple Choice
Based on the phase diagram shown below, which of the following statements are correct?
I. Sublimation occurs at a point in the transformation that falls along a straight line from point A to point F.
II. C and E represent points where the gas and liquid phases are in equilibrium.
III. ΔHvap can be measured at point B.
IV. Molecules at point D have a greater average kinetic energy than those at point F.
V. The temperature at point E is called the critical temperature of the compound. 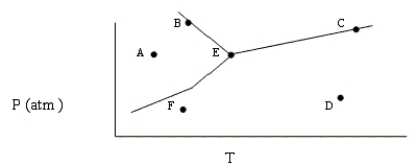
A) II, IV, V
B) I, II, IV
C) I, III, IV
D) II, V
E) I, II, III
Correct Answer:

Verified
Correct Answer:
Verified
Q91: Chromium metal crystallizes as a body-centered cubic
Q92: Identify the major attractive force in Cl<sub>2</sub>.<br>A)
Q93: Mn crystallizes in the same cubic unit
Q94: Given below are the temperatures at which
Q95: The unit cell in this two-dimensional crystal
Q97: Which of the following statements is true
Q98: Molecular complexity leads to lower viscosity.
Q99: The triple point of iodine is at
Q100: MnO has either a structure like NaCl
Q101: Of the three cubic unit cells, which