Multiple Choice
Below is a phase diagram for compound X. You wish to purify a sample of X that was collected at P = 1.0 atm and T = 100 by subliming it. In order to sublime the sample, you should 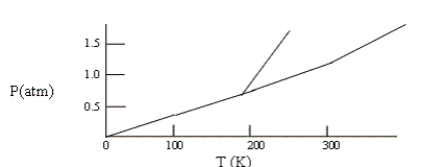
A) increase T to 300 K, keeping P = 1.0 atm.
B) abandon the attempt to sublime X.
C) lower P to 0.5 atm and then increase T to 200 K.
D) increase P to 1.5 atm and then increase T to 300 K.
E) increase T to 300 K and then lower P to 0.5 atm.
Correct Answer:

Verified
Correct Answer:
Verified
Q8: A certain compound with a molar mass
Q9: Which is generally larger, the heat of
Q10: The triple point of a substance is<br>A)
Q11: Brass is an example of<br>A) a superconductor.<br>B)
Q12: The density of the solid phase of
Q14: Identify the major attractive force in CaF<sub>2</sub>.<br>A)
Q15: What is the net number of face-centered
Q16: Which of the following chemical species has
Q17: The triple point of CO<sub>2</sub> is at
Q18: The molar volume of a certain form