Multiple Choice
The oxidation of Cr3+ to CrO42- can be accomplished using Ce4+ in a buffered solution. The following data were obtained: 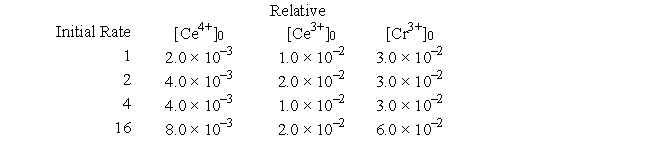
-Determine the order in the rate law of the species Cr3+.
A) -2
B) 3
C) 1
D) 2
E) -1
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q84: The following data were obtained for the
Q85: For the reaction 2N<sub>2</sub>O<sub>5</sub>(g) → 4NO<sub>2</sub>(g) +
Q86: For the reaction<br>2A + B → products<br>the
Q87: The following questions refer to the hypothetical
Q88: Which of the following statement is/are true
Q90: For which of the following is the
Q91: If the reaction 2HI → H<sub>2</sub> +
Q92: Consider the reaction<br>3A + B + C
Q93: For the reaction aA → products, select
Q94: A reaction represented by the equation<br>3O<sub>2</sub>(g) →