Multiple Choice
The reaction 2NO → N2 + O2 has the following rate law: 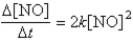 After a period of 2.0 × 103 s, the concentration of NO falls from an initial value of 2.8 × 10-3 mol/L to 2.0 × 10-4 mol/L. What is the rate constant, k?
After a period of 2.0 × 103 s, the concentration of NO falls from an initial value of 2.8 × 10-3 mol/L to 2.0 × 10-4 mol/L. What is the rate constant, k?
A) 4.0 × 10-4 M-1/s
B) 7.2 × 10-2 M-1/s
C) 1.7 × 10-4 M-1/s
D) 4.0 × 10-7 M-1/s
E) 3.6 × 10-2 M-1/s
Correct Answer:

Verified
Correct Answer:
Verified
Q1: For the reaction<br>2N<sub>2</sub>O<sub>5</sub>(g) → O<sub>2</sub>(g) + 4NO<sub>2</sub>(g)<br>the
Q2: For the reaction aA → products, select
Q4: The reaction 2NO<sub>2</sub> → 2NO + O<sub>2</sub>
Q5: The reaction of (CH<sub>3</sub>)<sub>3</sub>CBr with hydroxide ion
Q6: For the reaction aA → products, select
Q7: What is the rate law for the
Q8: The balanced equation for the reaction of
Q9: Use the following initial rate data for
Q10: Use the potential energy diagram shown to
Q11: The experimental rate law for the decomposition